electron configuration of fluorine|Chemistry of Fluorine (Z=9) : Clark 6.8: Electron Configurations - Chemistry LibreTexts Hoje, Sexta, 23/02/24. 00:10. Dinheiro Fácil. 02:00. Ajuste de Contas. 03:55. Movimento de Jesus. 06:00. Sex and the City 2.
PH0 · How to Write the Electron Configuration for Fluorine and F
PH1 · Fluorine Electronic Configuration and Distribution
PH2 · Fluorine Electron Configuration: 9 (Easy Step
PH3 · Fluorine Electron Configuration (F) with Orbital Diagram
PH4 · Fluorine Electron Configuration
PH5 · Fluorine
PH6 · Electron configuration of Fluorine
PH7 · Electron Configuration for Fluorine (F)
PH8 · Chemistry of Fluorine (Z=9)
PH9 · 6.8: Electron Configurations
Alluring 1.4K. Amateur 2.54M. Amateur Anal Sex 980K. Amateur Blowjob 665K. Amateur In Gangbang 29.3K. Amateur Interracial Sex 138K. Amateur Teen (18+) 6.6K. Amateur .
electron configuration of fluorine*******Chemistry of Fluorine (Z=9) - Chemistry LibreTextselectron configuration of fluorine6.8: Electron Configurations - Chemistry LibreTextsElectron Configuration for Fluorine (F) - UMD
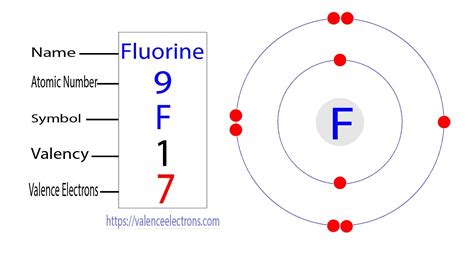
Electron Configuration for Fluorine (F) - UMDChemistry of Fluorine (Z=9) Learn how to write the electron configuration for fluorine using the period table or an electron configuration chart. See the video and the step-by-step tutorial for writing the electron configurations.
Learn the electron configuration of fluorine, its properties, uses, and history. Fluorine is the most reactive and electronegative element, with the symbol F and atomic number 9.Hun 30, 2023 — Learn about the properties, history, and reactions of fluorine, the most electronegative element in the periodic table. Fluorine has 5 valence electrons in the 2p level .Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. Boiling point .
Nob 14, 2013 — A step-by-step description of how to write the electron configuration for Fluorine (F). In order to write the F electron configuration we first need to know the number of .Learn how to construct the electron configuration of an element using the aufbau principle and Hund's rule. See examples of orbital diagrams and electron configurations for hydrogen to .Nob 17, 2022 — Electron configuration diagram of Fluorine is imposing the fact that two ‘s’ orbitals of the atom are filled with maximum capacity of 2 electrons but the p orbital is not filled with .Ene 21, 2021 — The electronic configuration for the Fluorine ion is 1s22s22p5 and in this configuration, Fluorine needs 1 electron so as to complete the 2p orbital. This 1 electron by the Fluorine will be acquired in this whole process of .Mar 2, 2023 — The electronic configuration of fluorine is: 1s2 2s2 2p5. Fluorine has 9 electrons arranged in two shells around the nucleus. The first shell contains two electrons, and the .
An Aufbau diagram showing the electron configuration of fluorine. Electron Configuration Standard Notation. A special type of notation is used to write an atom’s electron configuration. The notation describes the energy levels, .
Electrons and Electron Configuration. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Fluorine is 9. Each .Similarly, fluorine has the electron configuration 1s 2 2s 2 2p 5: Fluorine has one electron pair in the 1 s orbital, one electron pair in the 2 s orbital, and 2 electrons pairs with one unpaired electron in the 2 p orbital. When we reach neon, with Z = 10, we have filled the 2p subshell, giving a 1s 2 2s 2 2p 6 electron configuration:Hul 12, 2019 — Figure 2: Electronic configuration of fluorine. Fluorine is the most electronegative element because it has 5 electrons in its 2p shell. The optimal electron configuration of the 2p orbital contains 6 electrons, so since fluorine is so close to ideal electron configuration, the electrons are held very tightly to the nucleus.
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal .

Hul 12, 2022 — The EA of fluorine is –322 kJ/mol. When we add an electron to a fluorine atom to form a fluoride anion (F –), we add an electron to the n = 2 shell. The electron is attracted to the nucleus, but there is also significant repulsion from the other electrons already present in this small valence shell.
Mar 2, 2023 — The electronic configuration of Fluorine is 1s 2 2s 2 2p 5. Fluorine has one valence electron and, therefore, has a valency of one. FAQs. What is the electronic configuration of a fluorine atom? The electronic configuration of a fluorine atom is 1s 2 2s 2 2p 5. This means that there are two electrons in the first energy level, two electrons in .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal .
Fluorine's atomic electron configuration is 1s 2 2s 2 2p 5 (see Figure 2). Figure 2: Electronic configuration of fluorine. Fluorine is the most electronegative element because it has 5 electrons in its 2p shell. The optimal electron configuration of the 2p orbital contains 6 electrons, so since fluorine is so close to ideal electron .Abbreviated electronic configuration of Fluorine. The ground state abbreviated electronic configuration of Neutral Fluorine atom is [He] 2s2 2p5. The portion of Fluorine configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [He]. For atoms with many electrons, this notation can become lengthy and so an .Ene 21, 2021 — Further, the fluorine has the 9 atomic number and the electronic configuration of the Fluorine can be written as He] 2s2 2p5. Since the Fluorine holds the 7 electrons and this is why it needs to complete its octet within one .
The electronic configuration of Fluorine is 1 s 2 2 s 2 2 p 5. Suggest Corrections. 24. Similar questions. Q. The electronic structure (configuration) of fluorine can be written as 2,7. In a similar way give the electronic configuration of: Aluminium. Q.Similarly, fluorine has the electron configuration 1s 2 2s 2 2p 5: When we reach neon, with Z = 10, we have filled the 2p subshell, giving a 1s 2 2s 2 2p 6 electron configuration: Notice that for neon, as for helium, all the orbitals .Ago 14, 2020 — The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal .Hun 14, 2015 — The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save room, the configurations are in noble gas shorthand.This means part of the electron configuration has been replaced with the element symbol of the noble gas symbol.
Hul 20, 2022 — Similarly, fluorine has the electron configuration 1s 2 2s 2 2p 5: When we reach neon, with Z = 10, we have filled the 2p subshell, giving a 1s 2 2s 2 2p 6 electron configuration: Notice that for neon, as for helium, all the orbitals through the 2p level are completely filled. This fact is very important in dictating both the chemical .electron configuration of fluorine Chemistry of Fluorine (Z=9) Nob 17, 2022 — Fluorine electron configuration diagram Fluorine electron configuration notation. The notation of Fluorine’s electron configuration is found as [He] 1s 2 2s 2 2p 5 In the electron configuration of Fluorine the notation of electrons shells are used by general numbers such as 1, 2, and 3 and so on. The orbitals are denoted by alphabets s and p.Hun 30, 2023 — Therefore, fluorine has the highest electronegativity of all of the elements, indicated by its position on the periodic table. Its electron configuration is 1s 2 2s 2 2p 5. If fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in .
2 dias atrás · A Caixa Econômica Federal realizou nesta terça-feira, às 20h, o concurso 2.693 da Mega-Sena, com um prêmio de R$ 120.395.462,34. As dezenas sorteadas .
electron configuration of fluorine|Chemistry of Fluorine (Z=9)